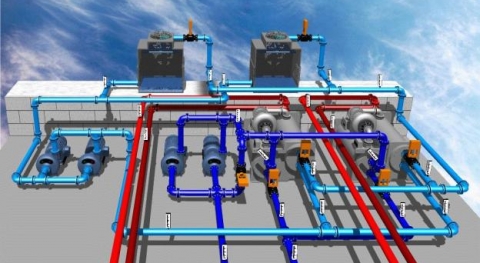
Maintenance Services Plumbing circulation (Cooling Tower, Chiller, Boiler, ...
The problem occurs in the coolant system closed circulation is caused by corrosion because there is no dispersion of salt concentration in the system.
The problem occurs in the coolant system closed circulation is caused by corrosion because there is no dispersion of salt concentration in the system.
In this system, the coolant rarely brain drain from the system, but only a small amount of water leak by pumping circulation ... The amount of leakage is generally only about 0.02% compared with the rate of circulation, the corrode easily accumulate in the system if such occurs.
Accordingly, corrosion inhibitor nitrite origin often used in high concentrations to prevent corrosion may occur. However, nitrites into nitrates altered bacterial nitrification and loss of efficiency as inhibitors. Bacterial treatment biocides used nitritation with nitrite inhibitor origin to prevent the consumption of nitrite in the water cooling system with water temperature closed from 10 to 40 ° C. The growth rate of the bacteria nitritation reduced when higher temperatures lower than 40 ° C and 10 ° C.
The problem occurs in the coolant system closed circulation is caused by corrosion because there is no dispersion of salt concentration in the system.
In this system, the coolant rarely brain drain from the system, but only a small amount of water leak by pumping circulation ... The amount of leakage is generally only about 0.02% compared with the rate of circulation, the corrode easily accumulate in the system if such occurs.
Accordingly, corrosion inhibitor nitrite origin often used in high concentrations to prevent corrosion may occur. However, nitrites into nitrates altered bacterial nitrification and loss of efficiency as inhibitors. Bacterial treatment biocides used nitritation with nitrite inhibitor origin to prevent the consumption of nitrite in the water cooling system with water temperature closed from 10 to 40 ° C. The growth rate of the bacteria nitritation reduced when higher temperatures lower than 40 ° C and 10 ° C.

When nitrite is applied to the system was corroded, they reduce the ammonium ions by iron and sometimes cooling water pH increases. When the pH exceeds 9.0 increases the corrosion of copper and copper alloys. To solve this problem, need to clean the system to reduce iron ions, using as borate pH buffer, using copper corrosion inhibitors such as triazole ... used in the system on.
In cooling water systems for marine engines, not just occur pitting corrosion but also feeds cylinder liners ... Moreover, the durability of metal devices is reduced by metal corrosion is also a serious problem. The use of corrosion inhibitor nitrite common origin for the system on because they are highly effective in preventing and reducing food pitting strength metal.
Inhibitors is sufficient to limit the corrosion although chlorine and sulfate ion concentration in the cooling water is increased by contamination from seawater. The addition of nitrite to increase the strength of cast steel in fresh water.
Corrosion inhibitors are denominated nitrite and economic efficiency, but they can cause environmental pollution because of high chemical oxygen demand and toxicity to aquatic life. Inhibitors have molybdate polymer gradually became popular as environmentally-friendly chemicals.
Inhibitors have been used for phosphate polymer slopes closed system for cooling in power plants ... the retention period than 10 days. Boiler treatment chemicals used effectively sealed coolant system - where there is no oxygen enters.
Maybe you care
Ý kiến bạn đọc: